By Henry Miller & Drew L. Kershen
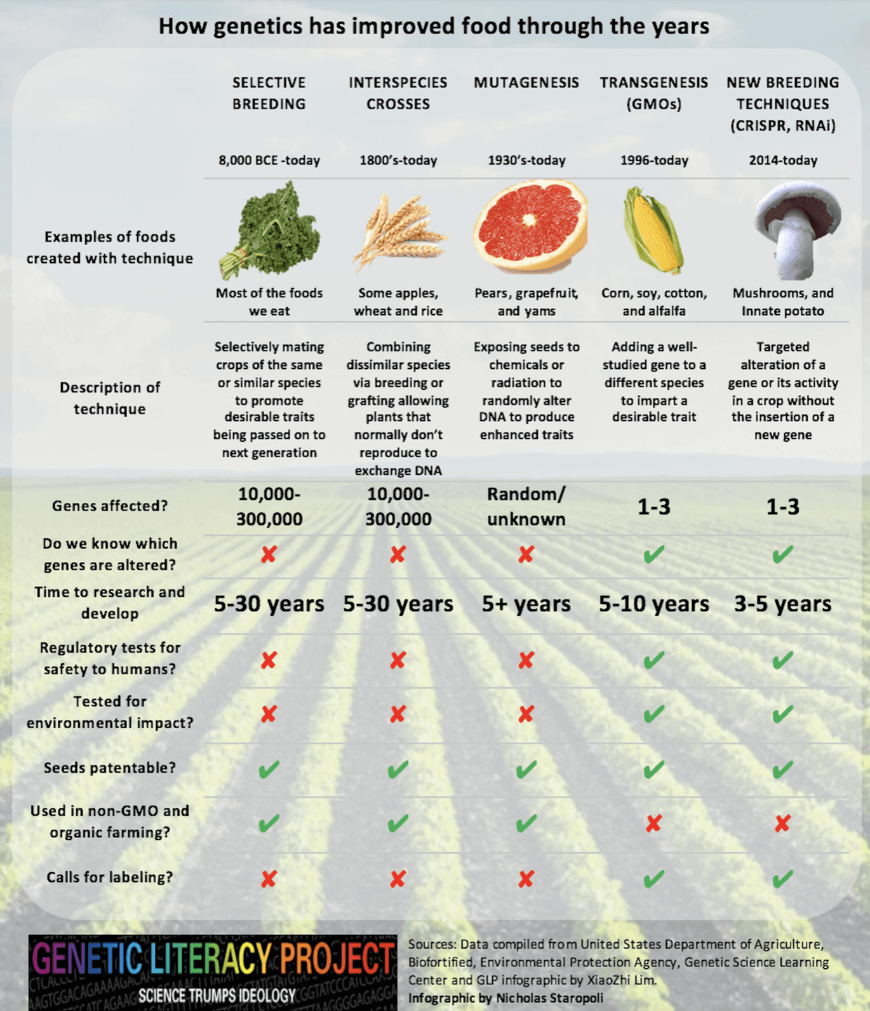
Although the public is almost completely unaware, today, more than three-quarters of all food crops have been directly and dramatically altered by humans using various processes, some relatively crude, by random experimentation, taking decades and even centuries, others extremely precise. Irrationally, and unrelated to safety and health, some products are highly regulated while others are virtually unregulated.
In fact, humans have been modifying the DNA of our food for thousands of years. That’s the very definition of agriculture. Early farmers (>10,000 years ago) used selective breeding to guide DNA changes (of which they were, of course, unaware) in crops to better suit our needs.
One of the most dramatic forms of genetic engineering, and among the least regulated, is a process called mutagenesis, which involves creating random mutations using harsh treatments that damage DNA. Approximately a hundred years ago, plant breeders began using harsh chemicals and/or radiation to randomly change, or mutate, the DNA of crops. About half of all crops today include mutagenesis as part of their pedigree.
These mutagens caused innumerable changes to the DNA, none of which was characterized or formally examined for safety. Nevertheless, problems were rare, and improvements were enormous.
The point we are making is that ancestral varieties bear little resemblance to the domesticated crops we eat today. There are many striking pictorial examples here, including these, of pre-modern crops that are now staples in our diet:
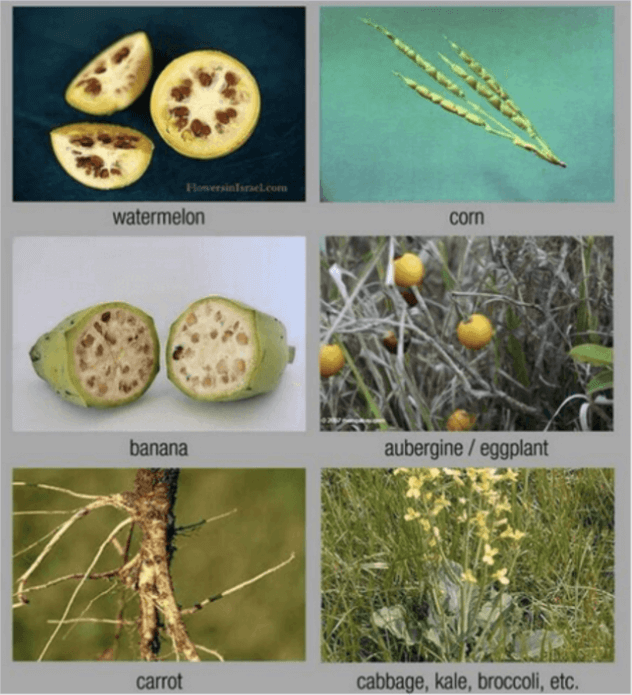
These evolutionary changes took hundreds or in some cases thousands of years of random breeding, with no concern about health issues, as plants can undergo thousands or even tens of thousands of mutations with little impact on humans. Random mutation breeding, despite creating many thousands of unknown mutations, has been shown to be safe with few missteps and minimal oversight. In fact, mutagenized products, from sweet tasting ruby red grapefruit to organic, pasta-quality durum wheat, were rolled out without testing or restrictions, and are sold as conventional and organic.

Science innovation makes the process more precise
Approximately 35 years ago agricultural scientists and plant breeders began to use recombinant DNA technology (“gene splicing”) to make far more predictable changes in the DNA in our crops. This molecular genetic engineering (GE) takes a gene with a known function — e.g., toxicity to certain insect pests — and moves it into a crop to transfer the desirable trait. This process is called “transgenesis,” but the resultant plant is referred to by its critics as a “genetically modified organism” or GMO—an appellation that is intended to be pejorative by implying that selective breeding and mutagenesis do not modify plants. Nonetheless, the highly precise and predictable process is revolutionary, by enabling the genetically engineered crop to protect itself from insect predation, which has allowed farmers around the world to reduce broad spectrum insecticide spraying by billions of pounds, a benefit to the natural environment, as well as to people.
It has been revolutionary for farmers and consumers. In Bangladesh, which introduced transgenic brinjal (eggplant) in 2014, developed license free by the government with technology donated by major biotechnology companies, farmers reduced pesticide spraying from 80 times a season to under five; yields and revenue jumped 20%; and medical care for applicators—mostly women and children—dropped six-fold. In poor countries, such technological innovations are lifesaving.
The most recent advance in this seamless continuum of genetic modification is genome editing (also called gene editing), which is accomplished by technologies that allow genetic material to be added, removed, or altered at specific locations in the genome. The best known of these is CRISPR-Cas9, which is short for Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR-associated protein 9. The CRISPR-Cas9 system is faster, cheaper, more precise, and more efficient than earlier genome editing methods. Here is a gene editing FAQ developed by the Cornell Alliance for Science.

A potentially revolutionary application of CRISPR is the creation of a “gene drive,” a genetic modification designed to spread through a population at higher-than-normal rates of inheritance. It uses CRISPR and small segments of RNA to alter or silence a specific gene, or insert a new one. In the next generation of the organism, the whole drive copies itself onto its partner chromosome so that the genome no longer contains the natural version of the chosen gene, but instead has two copies of the gene drive. In this way, the genetic change is passed on to up to 100% of offspring, rather than around 50%.
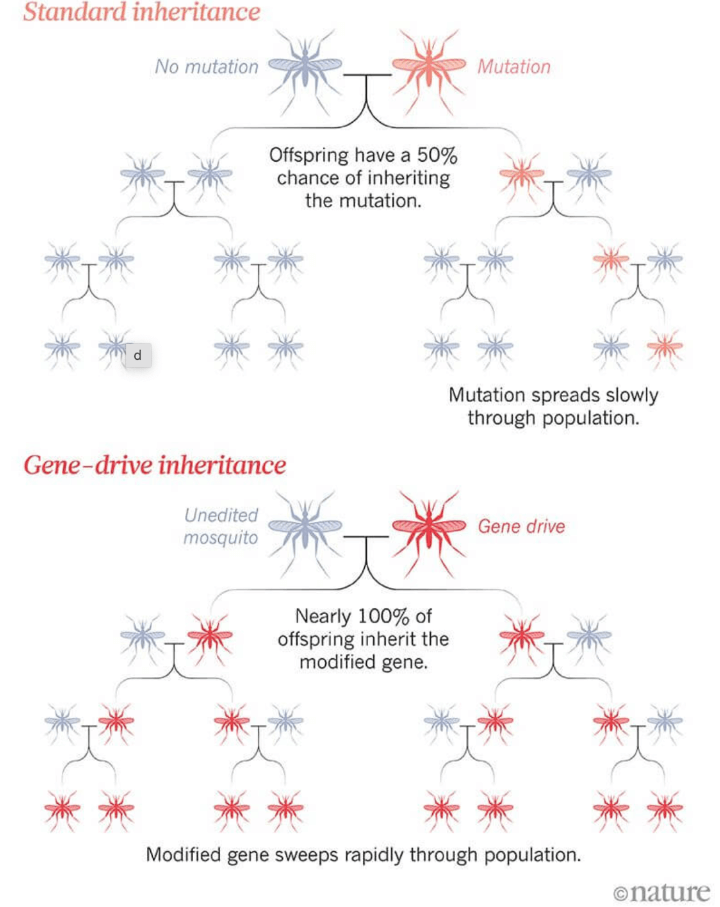
Because gene drives have the potential to be very powerful, possibly altering or eradicating entire populations, they can affect whole ecosystems. In that way, they differ from other narrower applications of genetic engineering, new or old, and must be tested incrementally and judiciously.
Gene editing, gene drives and ‘ethical’ oversight
The CRISPR Journal recently published A Code of Ethics for Gene Drive Research, prepared by scientists and health experts involved in gene-editing mosquitoes. Similarly, the Coalition for Responsible Gene Editing in Agriculture (coordinated by the Center for Food Integrity) released a Framework for Responsible Use of Gene Editing in Agriculture in February 2022. In crafting the Code and the Framework, respectively, scientists and product developers sought to engage responsibly with the public in science communication and education. They are trying to gain the public’s trust for the use of various genetic techniques in health, agriculture and food.
The principles adopted for the gene drive Code and the Framework for gene editing in agriculture are similar. The former, for example, cites the importance of scientific responsibility, ecological stewardship, public engagement, and fair and equitable benefit-sharing. We generally support those goals, in part because gene drives exhibit qualities that can be drastic and irreversible.
However, the Framework treats gene editing as though it were a technology giving rise to unique products unlike those previously seen or used — an approach that flagrantly ignores the seamless evolution of genetic engineering discussed above: that gene editing is part of a seamless continuum of genetic modification and that it is more precise and predictable than pre-molecular techniques; and that regulatory requirements already exist for products that may pose significant risks — pharmaceuticals, medical devices, pesticides, noxious weeds, and so on.
One illustration of how the Framework treats gene editing in an inappropriate sui generis way is this claim:
Prior to commercialization, conduct a market and trade assessment appropriate to the gene edited product that anticipates and considers the potential domestic and international impacts on relevant stakeholders up and down the value chain.
Should the fact that a higher-yielding wheat or corn variety that disadvantages organic farmers (because genetically engineered varieties are proscribed in organic practices) be taken into consideration? Or if bacteria were gene edited such that they became a source of cinnamon (similar to the synthesis of human insulin in genetically engineered E. coli.), before marketing their product, should the producers be required to analyze the “impacts on relevant stakeholders?” (The spice is native to Sri Lanka, the neighboring Malabar Coast of India, and Myanmar, and is also cultivated in South America and the West Indies.)
We believe such a proposal is ridiculous, not unlike requiring the manufacturers of electric vehicles to analyze the possible impacts of their products on oil refiners and the makers of gasoline-powered vehicles.
The authors of the Code and the Framework appear to embrace two underlying assumptions:
- Scientists and product developers have the primary responsibility for informing and educating the public, which they have so far failed to do adequately; and
- Public trust will emerge if proper educational efforts and information exists.
This is an “educational deficit” model for seeking public trust — a plausible and useful model. However, history repudiates the view that it explains the problem of public trust of genetic modification.
From our years of experience in analyzing the development, use, and regulation of the products of genetic engineering for healthcare, agriculture, and food security, we have found that public distrust —or at the very least, skepticism — has been driven by two other factors: competing economic interests that have a monetary incentive to demonize genetic techniques; and the politicization of science for ideological reasons. We discuss them below.
Ideology, economics and politics of genetic engineering
First, the organic agriculture and food movement has a direct and potent economic interest in defaming modern genetic engineering, which has the potential to transform the yield gap between conventional and organic agriculture into a chasm. The organic movement has loudly and consistently proclaimed that they will refuse to accept gene-edited products under their rubric.
In 2019, for example when U.S. Department of Agriculture suggested that gene editing should be considered for use in organic food production, The Organic Trade Association issued a statement that said it “maintains its long-held position that any gene editing techniques not be allowed in organic production.”
They maintain that position despite the fact that the mutations that occur through directed gene editing are indistinguishable from what occurs “in nature” — as if that were at all relevant, considering that thousands of years of genetic modification, including mutagenesis, are allowed under archaic organic rules.
Politics distorts the marketplace
Fear sells, and sells even better at premium prices. Abetted by environmental activists and some prominent restaurateurs and food writers, the movement propagandizes to convince the public that molecular genetic techniques are somehow unnatural and harmful. They have been largely successful in that misbegotten mission.
Allied with those who have competing economic interests are others who have ideological reasons — opposition to corporations and biotechnology and embrace of a European-inspired ‘precautionary principle’ mindset — for attacking science and molecular genetic techniques.
This combined opposition uses political influence and political power to impede the use of molecular genetic engineering with unneeded and burdensome statutory and regulatory requirements. It purposefully promotes and supports trade and marketing restrictions and overregulation, both nationally and internationally, of safe and beneficial products from superior technologies.
No better examples of this combined opposition exist than the delays and bans on nutritious foods for the poor such as genetically engineered pest-resistant eggplant in India and vitamin A-fortified Golden Rice in the Philippines and elsewhere in Asia.
Beyond the commercial and ideological opposition, geopolitical campaigns of misinformation and falsehoods also purposefully manipulate public opinion. As science writer Dan Vergano reported, Russia created something called the Factor GMO Project, an elaborate campaign (which eventually evaporated), to create doubts and spread misinformation about genetic engineering. In various venues, the massive Russian propaganda machine disparages not only U.S.-dominant technologies such as molecular genetic engineering and fracking, but also American-made COVID-19 vaccines. Russia also funds Western NGOs and others, whom Lenin termed “useful idiots”, to advance their agenda.
The developers and purveyors of new products must communicate frankly and honestly with the public, to be sure, but there is no justification for holding certain classes of products to an unwarranted higher standard — such as the gene editing Framework’s “social considerations,” for “reassurance” of the public. Doing so disrupts free markets, raises R&D costs, impedes innovation, confuses consumers, and is detrimental to society. If anything, a superfluous higher standard fans public concerns about a safe, superior technology. As Barbara Keating-Edh, representing the consumer group Consumer Alert, testified before the U.S. National Biotechnology Policy Board:
For obvious reasons, the consumer views the technologies that are most regulated to be the least safe ones. Heavy involvement by government, no matter how well intended, inevitably sends the wrong signals. Rather than ensuring confidence, it raises suspicion and doubt” [emphasis in original]. (Keating-Edh, B. Statement before the National Biotechnology Policy Board (20 September 1991), cited in Biotechnology Law Report, March-April 1993, 12 (2); 127-182.)
Labelling
A U.S. Department of Agriculture labeling regulation that went into effect at the beginning of 2022 illustrates how policymakers tie themselves into knots when they forsake science in order to provide consumers with meaningless information. It is a masterpiece of incoherence.

For example, it does not require the labeling of highly refined ingredients from bioengineered crops “if the food does not contain detectable genetically modified material,” but it allows manufacturers to make voluntary disclosures on such products. That leaves open the possibility that two identical bottles of corn oil on the supermarket shelf, derived from the same harvested field and identical in processing and quality, could be labeled differently – one as “derived from bioengineering” and the other without that designation — but both would comply with the regulation. The rule does not permit “may contain” disclosures.
“Detectable” is also problematic. Technologies evolve and become ever more sensitive, so a single, detectable molecule of “genetically modified material” would make a food “bioengineered.” This is an invitation to frivolous litigation over what is “detectable.”
Under the enabling statute, labels are mandatory only if the food is also covered by food labels administered by the FDA or USDA’s Food Safety Inspection Service. Thus, the rule requires meticulous statutory interfaces that may make food lawyers cross-eyed or wealthy – or both.
USDA wrote this regulation in response to a Congressional statute adopted in 2016. We have previously commented more fully on its shortcomings in Nature Biotechnology here.
Finally, there is the kicker, which USDA admitted in the comments accompanying the final regulation: In spite of costs of hundreds of millions of dollars, the bioengineered label “is not expected to have any benefits to human health or the environment” [emphasis added].
This is a stunning example of the triumph of political and ideological considerations over common sense about genetic techniques for agricultural advances.
Going forward
We suggest that the Code and Framework would be improved if they affirmed two principles for communicating about genetic techniques — courage and integrity; the courage to resist opponents whose clamor seeks to misinform, impugn, and overregulate science and technology; and the integrity to firmly and forthrightly promote the science and scientists whose work benefits individuals and society. Both the Code and the Framework will need these virtues to withstand the misinformation, falsehoods, and ideological attacks that gene editing and gene drives will surely continue to encounter.
Dr. Henry I. Miller, a physician and molecular biologist, is a senior fellow at the Pacific Research Institute. He was the founding director of the FDA’s Office of Biotechnology and a Consulting Professor at Stanford University’s Institute for International Studies. Find Henry on Twitter @henryimiller
Mr. Drew L. Kershen is a professor emeritus at the University of Oklahoma College of Law.
Public trust, safety, and genetic engineering
Pacific Research Institute
By Henry Miller & Drew L. Kershen
Although the public is almost completely unaware, today, more than three-quarters of all food crops have been directly and dramatically altered by humans using various processes, some relatively crude, by random experimentation, taking decades and even centuries, others extremely precise. Irrationally, and unrelated to safety and health, some products are highly regulated while others are virtually unregulated.
In fact, humans have been modifying the DNA of our food for thousands of years. That’s the very definition of agriculture. Early farmers (>10,000 years ago) used selective breeding to guide DNA changes (of which they were, of course, unaware) in crops to better suit our needs.
One of the most dramatic forms of genetic engineering, and among the least regulated, is a process called mutagenesis, which involves creating random mutations using harsh treatments that damage DNA. Approximately a hundred years ago, plant breeders began using harsh chemicals and/or radiation to randomly change, or mutate, the DNA of crops. About half of all crops today include mutagenesis as part of their pedigree.
These mutagens caused innumerable changes to the DNA, none of which was characterized or formally examined for safety. Nevertheless, problems were rare, and improvements were enormous.
The point we are making is that ancestral varieties bear little resemblance to the domesticated crops we eat today. There are many striking pictorial examples here, including these, of pre-modern crops that are now staples in our diet:
These evolutionary changes took hundreds or in some cases thousands of years of random breeding, with no concern about health issues, as plants can undergo thousands or even tens of thousands of mutations with little impact on humans. Random mutation breeding, despite creating many thousands of unknown mutations, has been shown to be safe with few missteps and minimal oversight. In fact, mutagenized products, from sweet tasting ruby red grapefruit to organic, pasta-quality durum wheat, were rolled out without testing or restrictions, and are sold as conventional and organic.
Science innovation makes the process more precise
Approximately 35 years ago agricultural scientists and plant breeders began to use recombinant DNA technology (“gene splicing”) to make far more predictable changes in the DNA in our crops. This molecular genetic engineering (GE) takes a gene with a known function — e.g., toxicity to certain insect pests — and moves it into a crop to transfer the desirable trait. This process is called “transgenesis,” but the resultant plant is referred to by its critics as a “genetically modified organism” or GMO—an appellation that is intended to be pejorative by implying that selective breeding and mutagenesis do not modify plants. Nonetheless, the highly precise and predictable process is revolutionary, by enabling the genetically engineered crop to protect itself from insect predation, which has allowed farmers around the world to reduce broad spectrum insecticide spraying by billions of pounds, a benefit to the natural environment, as well as to people.
It has been revolutionary for farmers and consumers. In Bangladesh, which introduced transgenic brinjal (eggplant) in 2014, developed license free by the government with technology donated by major biotechnology companies, farmers reduced pesticide spraying from 80 times a season to under five; yields and revenue jumped 20%; and medical care for applicators—mostly women and children—dropped six-fold. In poor countries, such technological innovations are lifesaving.
The most recent advance in this seamless continuum of genetic modification is genome editing (also called gene editing), which is accomplished by technologies that allow genetic material to be added, removed, or altered at specific locations in the genome. The best known of these is CRISPR-Cas9, which is short for Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR-associated protein 9. The CRISPR-Cas9 system is faster, cheaper, more precise, and more efficient than earlier genome editing methods. Here is a gene editing FAQ developed by the Cornell Alliance for Science.
A potentially revolutionary application of CRISPR is the creation of a “gene drive,” a genetic modification designed to spread through a population at higher-than-normal rates of inheritance. It uses CRISPR and small segments of RNA to alter or silence a specific gene, or insert a new one. In the next generation of the organism, the whole drive copies itself onto its partner chromosome so that the genome no longer contains the natural version of the chosen gene, but instead has two copies of the gene drive. In this way, the genetic change is passed on to up to 100% of offspring, rather than around 50%.
Because gene drives have the potential to be very powerful, possibly altering or eradicating entire populations, they can affect whole ecosystems. In that way, they differ from other narrower applications of genetic engineering, new or old, and must be tested incrementally and judiciously.
Gene editing, gene drives and ‘ethical’ oversight
The CRISPR Journal recently published A Code of Ethics for Gene Drive Research, prepared by scientists and health experts involved in gene-editing mosquitoes. Similarly, the Coalition for Responsible Gene Editing in Agriculture (coordinated by the Center for Food Integrity) released a Framework for Responsible Use of Gene Editing in Agriculture in February 2022. In crafting the Code and the Framework, respectively, scientists and product developers sought to engage responsibly with the public in science communication and education. They are trying to gain the public’s trust for the use of various genetic techniques in health, agriculture and food.
The principles adopted for the gene drive Code and the Framework for gene editing in agriculture are similar. The former, for example, cites the importance of scientific responsibility, ecological stewardship, public engagement, and fair and equitable benefit-sharing. We generally support those goals, in part because gene drives exhibit qualities that can be drastic and irreversible.
However, the Framework treats gene editing as though it were a technology giving rise to unique products unlike those previously seen or used — an approach that flagrantly ignores the seamless evolution of genetic engineering discussed above: that gene editing is part of a seamless continuum of genetic modification and that it is more precise and predictable than pre-molecular techniques; and that regulatory requirements already exist for products that may pose significant risks — pharmaceuticals, medical devices, pesticides, noxious weeds, and so on.
One illustration of how the Framework treats gene editing in an inappropriate sui generis way is this claim:
Should the fact that a higher-yielding wheat or corn variety that disadvantages organic farmers (because genetically engineered varieties are proscribed in organic practices) be taken into consideration? Or if bacteria were gene edited such that they became a source of cinnamon (similar to the synthesis of human insulin in genetically engineered E. coli.), before marketing their product, should the producers be required to analyze the “impacts on relevant stakeholders?” (The spice is native to Sri Lanka, the neighboring Malabar Coast of India, and Myanmar, and is also cultivated in South America and the West Indies.)
We believe such a proposal is ridiculous, not unlike requiring the manufacturers of electric vehicles to analyze the possible impacts of their products on oil refiners and the makers of gasoline-powered vehicles.
The authors of the Code and the Framework appear to embrace two underlying assumptions:
This is an “educational deficit” model for seeking public trust — a plausible and useful model. However, history repudiates the view that it explains the problem of public trust of genetic modification.
From our years of experience in analyzing the development, use, and regulation of the products of genetic engineering for healthcare, agriculture, and food security, we have found that public distrust —or at the very least, skepticism — has been driven by two other factors: competing economic interests that have a monetary incentive to demonize genetic techniques; and the politicization of science for ideological reasons. We discuss them below.
Ideology, economics and politics of genetic engineering
First, the organic agriculture and food movement has a direct and potent economic interest in defaming modern genetic engineering, which has the potential to transform the yield gap between conventional and organic agriculture into a chasm. The organic movement has loudly and consistently proclaimed that they will refuse to accept gene-edited products under their rubric.
In 2019, for example when U.S. Department of Agriculture suggested that gene editing should be considered for use in organic food production, The Organic Trade Association issued a statement that said it “maintains its long-held position that any gene editing techniques not be allowed in organic production.”
They maintain that position despite the fact that the mutations that occur through directed gene editing are indistinguishable from what occurs “in nature” — as if that were at all relevant, considering that thousands of years of genetic modification, including mutagenesis, are allowed under archaic organic rules.
Politics distorts the marketplace
Fear sells, and sells even better at premium prices. Abetted by environmental activists and some prominent restaurateurs and food writers, the movement propagandizes to convince the public that molecular genetic techniques are somehow unnatural and harmful. They have been largely successful in that misbegotten mission.
Allied with those who have competing economic interests are others who have ideological reasons — opposition to corporations and biotechnology and embrace of a European-inspired ‘precautionary principle’ mindset — for attacking science and molecular genetic techniques.
This combined opposition uses political influence and political power to impede the use of molecular genetic engineering with unneeded and burdensome statutory and regulatory requirements. It purposefully promotes and supports trade and marketing restrictions and overregulation, both nationally and internationally, of safe and beneficial products from superior technologies.
No better examples of this combined opposition exist than the delays and bans on nutritious foods for the poor such as genetically engineered pest-resistant eggplant in India and vitamin A-fortified Golden Rice in the Philippines and elsewhere in Asia.
Beyond the commercial and ideological opposition, geopolitical campaigns of misinformation and falsehoods also purposefully manipulate public opinion. As science writer Dan Vergano reported, Russia created something called the Factor GMO Project, an elaborate campaign (which eventually evaporated), to create doubts and spread misinformation about genetic engineering. In various venues, the massive Russian propaganda machine disparages not only U.S.-dominant technologies such as molecular genetic engineering and fracking, but also American-made COVID-19 vaccines. Russia also funds Western NGOs and others, whom Lenin termed “useful idiots”, to advance their agenda.
The developers and purveyors of new products must communicate frankly and honestly with the public, to be sure, but there is no justification for holding certain classes of products to an unwarranted higher standard — such as the gene editing Framework’s “social considerations,” for “reassurance” of the public. Doing so disrupts free markets, raises R&D costs, impedes innovation, confuses consumers, and is detrimental to society. If anything, a superfluous higher standard fans public concerns about a safe, superior technology. As Barbara Keating-Edh, representing the consumer group Consumer Alert, testified before the U.S. National Biotechnology Policy Board:
Labelling
A U.S. Department of Agriculture labeling regulation that went into effect at the beginning of 2022 illustrates how policymakers tie themselves into knots when they forsake science in order to provide consumers with meaningless information. It is a masterpiece of incoherence.
For example, it does not require the labeling of highly refined ingredients from bioengineered crops “if the food does not contain detectable genetically modified material,” but it allows manufacturers to make voluntary disclosures on such products. That leaves open the possibility that two identical bottles of corn oil on the supermarket shelf, derived from the same harvested field and identical in processing and quality, could be labeled differently – one as “derived from bioengineering” and the other without that designation — but both would comply with the regulation. The rule does not permit “may contain” disclosures.
“Detectable” is also problematic. Technologies evolve and become ever more sensitive, so a single, detectable molecule of “genetically modified material” would make a food “bioengineered.” This is an invitation to frivolous litigation over what is “detectable.”
Under the enabling statute, labels are mandatory only if the food is also covered by food labels administered by the FDA or USDA’s Food Safety Inspection Service. Thus, the rule requires meticulous statutory interfaces that may make food lawyers cross-eyed or wealthy – or both.
USDA wrote this regulation in response to a Congressional statute adopted in 2016. We have previously commented more fully on its shortcomings in Nature Biotechnology here.
Finally, there is the kicker, which USDA admitted in the comments accompanying the final regulation: In spite of costs of hundreds of millions of dollars, the bioengineered label “is not expected to have any benefits to human health or the environment” [emphasis added].
This is a stunning example of the triumph of political and ideological considerations over common sense about genetic techniques for agricultural advances.
Going forward
We suggest that the Code and Framework would be improved if they affirmed two principles for communicating about genetic techniques — courage and integrity; the courage to resist opponents whose clamor seeks to misinform, impugn, and overregulate science and technology; and the integrity to firmly and forthrightly promote the science and scientists whose work benefits individuals and society. Both the Code and the Framework will need these virtues to withstand the misinformation, falsehoods, and ideological attacks that gene editing and gene drives will surely continue to encounter.
Dr. Henry I. Miller, a physician and molecular biologist, is a senior fellow at the Pacific Research Institute. He was the founding director of the FDA’s Office of Biotechnology and a Consulting Professor at Stanford University’s Institute for International Studies. Find Henry on Twitter @henryimiller
Mr. Drew L. Kershen is a professor emeritus at the University of Oklahoma College of Law.
Nothing contained in this blog is to be construed as necessarily reflecting the views of the Pacific Research Institute or as an attempt to thwart or aid the passage of any legislation.